Uncovering the “why” behind your symptoms is key to unlocking true healing. The “why” is found within your life story. Our practice was created to give you and your practitioner the time necessary to uncover the intricacies of your health journey.
Your health journey starts the moment your life begins. Here are some questions & reasons your functional medicine provider may ask you when starting to uncover the “why’s” behind physical symptoms.
“In the United States, about half the C-sections we do appear to be avoidable. This study is the first to estimate the potential population-wide harms of this trend to mothers over the long term” – co-author Dr. Neel Shah, who leads the Delivery Decisions Initiative at Ariadne Labs
Did your mother give birth to you vaginally or by C-Section?
A caesarean section (CS) can be a life-saving intervention when medically indicated, but this procedure can also lead to short-term and long-term health effects for women and children. Given the increasing use of CS, particularly without medical indication, an increased understanding of its health effects on women and children has become crucial.
There is emerging evidence that babies born by CS have different hormonal, physical, bacterial, and medical exposures, and that these exposures can subtly alter neonatal physiology. Short-term risks of CS include altered immune development, an increased likelihood of allergy, atopy, and asthma, and reduced intestinal gut microbiome diversity.
The persistence of these risks into later life is less well investigated, although an association between CS use and greater incidence of late childhood obesity and asthma are frequently reported. There are few studies that focus on the effects of CS on cognitive and educational outcomes. The study, published in the Aug 9 issue of JAMA Surgery, was led by researchers at Aalborg University in Denmark and at Ariadne Labs in Boston. More than 23 million women across the globe have C-sections each year, making it the most common surgery in the world by far. More than one million women have hysterectomies later in life to remove their uterus, most often because of pain and/or bleeding.
Understanding potential mechanisms that link CS with childhood outcomes, such as the role of the developing neonatal microbiome, has potential to inform practitioners so they can better aid in restoring patients to optimal health.
 What were some of your biggest losses, traumas & life events?
What were some of your biggest losses, traumas & life events?
Providers need to understand how trauma can affect treatment presentation, engagement, and the outcome of physical, mental, emotional, and behavioral health services. Trauma, including one-time, multiple, or long-lasting repetitive events, affects everyone differently.
Immediate and Delayed Reactions to Trauma
Some individuals may clearly display criteria associated with posttraumatic stress disorder (PTSD), but many more individuals will exhibit resilient responses or brief subclinical symptoms or consequences that fall outside of diagnostic criteria. The impact of trauma can be subtle, insidious, or outright destructive. How an event affects an individual depends on many factors, including characteristics of the individual, the type and characteristics of the event(s), developmental processes, the meaning of the trauma, and sociocultural factors.
From sleep disorders to food addictions/coping mechanisms, working with a provider to recognize traumas & triggers, create safe environments, and aid in re-regulating nervous systems in healthy & helpful manners can help restore mental, emotional, and physical well-being.
 Were you exposed to antibiotics early in life?
Were you exposed to antibiotics early in life?
During birth, a relatively sterile unborn child becomes a newborn coated with microbes on every surface. This collection of bacteria, archaea, viruses, and fungi found in and on the human body is called the microbiota. The collective genomes of the microbiota are considered to be the metagenome, and the totality of the microbiota, metagenome, and their interactions is the microbiome. The microbiota has many critical functions including protection from pathogens, development and maintenance of the immune system, and helping the host access nutrients in food. The gut microbiota has been of particular interest, as perturbations of this community have been linked to disease states including autoimmune disease and neurological disorders. Antibiotics have consistently been shown to change the gut microbiome in humans and animals.
The infant gut microbiota increases in diversity and richness while becoming more stable over time, especially once solid foods are introduced into the diet, until the community resembles an adult-like state at around three years old.
Since the gut microbiota is important in host immune development, nutrient absorption, and protection from pathogens, changes in the community composition could have deleterious effects on the host. The spectrum of diseases for which an altered gut microbiota has been implicated is quite broad. Several excellent reviews have focused on the relationship between the microbiota and host immunity. One critical aspect of this field is that T cell populations in the gut can be influenced by the microbiota and its metabolites. One of the most studied groups of bacterial metabolites, short-chain fatty acids, have been shown to exert epigenetic regulation of transcription factor genes to influence regulatory T cells in the gut. Changes in T cell populations are one mechanism by which alterations in the gut microbiota may be contributing to autoimmune diseases including inflammatory bowel disease (IBD), asthma, allergies, arthritis, and multiple sclerosis (MS) Thirteen studies, including a total of 527 504 children, were included in a systematic review and concluded exposure to antibiotics in infancy was associated with an increased odds ratio (OR) of childhood overweight and obesity.
Understanding your unique gut microbiome is vital to creating an environment that allows the rest of your body to function properly & thrive!
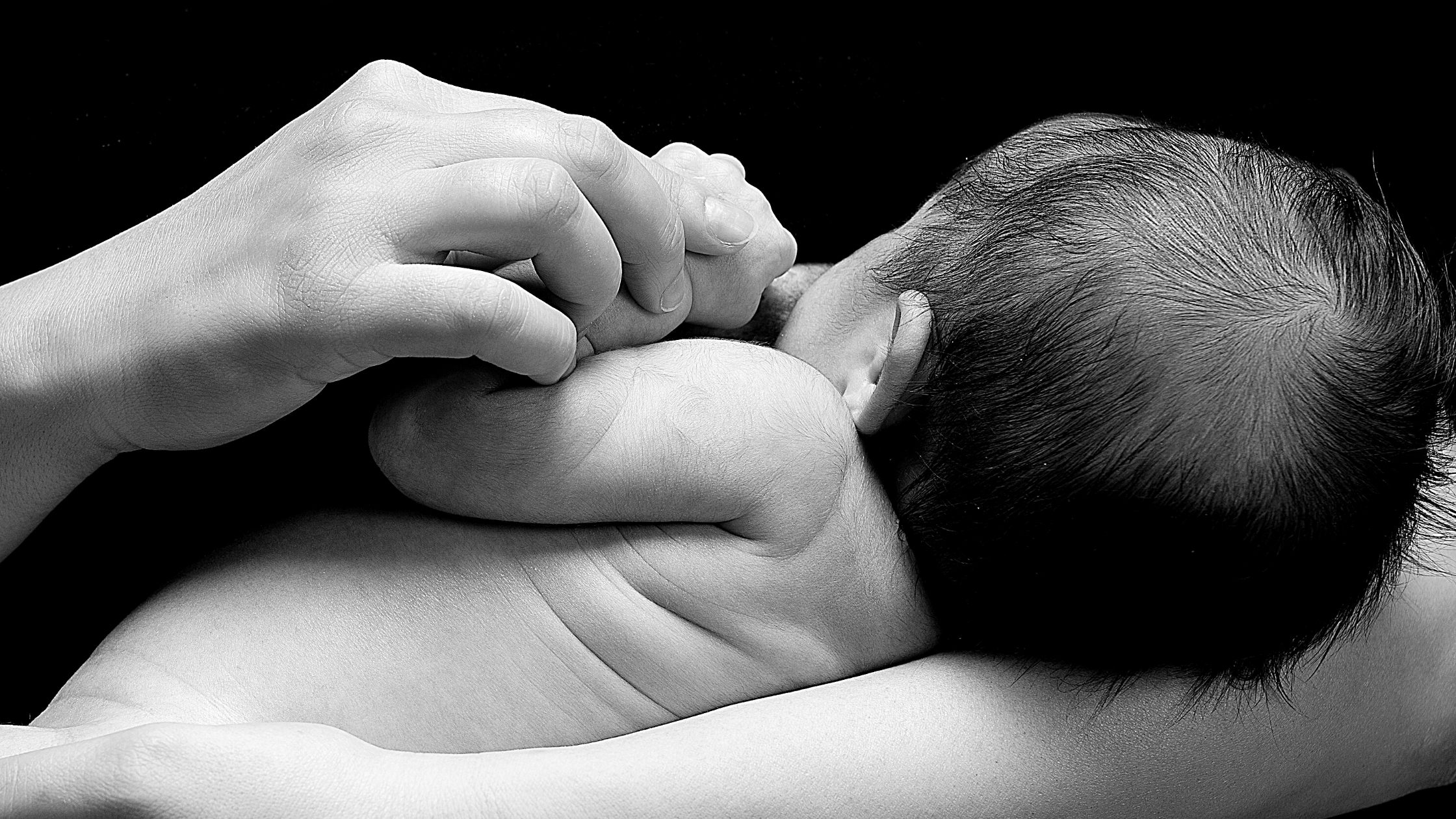 Were you breastfed as a child?
Were you breastfed as a child?
Health outcomes in developed countries differ substantially for mothers and infants who formula feed compared with those who breastfeed. For infants, not being breastfed is associated with an increased incidence of infectious morbidity, as well as elevated risks of childhood obesity, type 1 and type 2 diabetes, leukemia, and sudden infant death syndrome. For mothers, failure to breastfeed is associated with an increased incidence of premenopausal breast cancer, ovarian cancer, retained gestational weight gain, type 2 diabetes, myocardial infarction, and the metabolic syndrome.
Compared with breastfed infants, formula-fed infants face higher risks of infectious morbidity in the first year of life. These differences in health outcomes can be explained, in part, by specific and innate immune factors present in human milk.1 Plasma cells in the mother’s bronchial tree and intestine migrate to the mammary epithelium and produce IgA antibodies specific to antigens in the mother-infant dyad’s immediate surroundings, providing specific protection against pathogens in the mother’s environment.2 In addition, innate immune factors in milk provide protection against infection.
Not breastfeeding or weaning prematurely is associated with health risks for mothers as well as for infants. Epidemiologic data suggest that women who do not breastfeed face higher risk of breast cancer and ovarian cancer, as well as obesity, type 2 diabetes, metabolic syndrome, and cardiovascular disease. As in the pediatric literature, most evidence arises from observational studies, which are subject to confounding by other health behaviors. For maternal health outcomes, associations are generally reported according to lifetime duration across all pregnancies, rather than duration of feeding for each pregnancy.
Given the compelling evidence for differences in health outcomes, breastfeeding should be acknowledged as the biologic norm for infant feeding. Physician counseling, clinics, and hospital practices should be aligned to ensure that the breastfeeding mother-infant dyad has the best chance for a long, successful breastfeeding experience. And, if it is not an option for a mother, practitioners should be informed so that they can best support the mother and infant through supplementary practices.
 Did you achieve puberty at an early age?
Did you achieve puberty at an early age?
Adolescence is increasingly recognized as a critical period in the life course, a time when rapid development of the brain, body, and behaviors opens a window of opportunity for interventions that may affect health throughout life.
Puberty results in very rapid somatic growth, brain development, sexual maturation, and attainment of reproductive capacity. It is accompanied by final maturation of multiple organ systems and major changes in the central nervous system and in psychosocial behavior (Patton and Viner 2007).
A range of social determinants of health arise in adolescence, with peers, schools, and eventually the workplace becoming strong determinants of health and well-being as the influence of the family wanes (Viner and others 2012). These social changes are apparent even in traditional or more sociocentric cultures. More than half of the top 10 risk factors identified in the Global Burden of Disease study (GBD 2013 Risk Factors Collaborators 2015) are largely determined during adolescence.
Adolescence is also a time when young people may modify or alter the pathways to adult health or illness (Viner and others 2012). Early life experiences may reinforce both good and poor trajectories. Similarly, resilience during adolescence may improve outcomes for young people born into adversity. The transfer from primary to secondary school, sexual debut, and entry into the labor market may be critical points for preventing the accumulation of health risk (Viner and others 2012).
Adolescence is a time of great developmental plasticity and risk for the onset of a range of disorders that can carry a high burden of disease throughout the lifespan. It offers a critical developmental window of opportunity for intervention and prevention. Puberty and brain development during adolescence are responsible for dramatic shifts in burden of disease, away from childhood conditions toward injuries and emerging noncommunicable diseases. Knowledge of the unique developmental processes that characterize adolescence and the role they play in both risk and opportunity during this phase of life is expanding rapidly. What remains is the task of translating this knowledge into intervention and prevention methods that target modifiable, developmentally sensitive mechanisms to maximize the effectiveness of intervention approaches during this phase of life.
 What is your work environment like?
What is your work environment like?
Chronic, sustained exposure to stressful working conditions can result in a variety of long term health problems, including: Cardiovascular disease, Musculoskeletal disorders, & Psychological disorders.
Objectively assessed job demands were significantly associated with blood pressure and Cortisol levels. The model also predicted elevations in physiological responses after individuals left work, suggesting that potentially health-impairing reactions to jobs that have high demands and low controllability might carry over to home settings and thus pose a high risk of long-term health impairment. The results have implications for the role of personal control in occupational stress.
The goal is to create conditions that do not trigger the biologic and behavioral pathways to disease & disorders. Creating and implementing practices and environmental support requires leadership and strong cross-functional collaboration outside of the traditional structures for workplace health, safety and wellness.
Knowing the impact your environment has on your overall well-being is crucial to learning how to aid your body through nutrients, adaptogens, and healthy stress management practices.
At Kingdom Health & Wellness…
Schedule an appointment
Champagne N, et al. Obesity/Overweight and the Role of Working Conditions: A Qualitative Participatory Investigation. Univ. Mass. Lowell, Oct 2012. www.uml.edu/research/centers/CPH-NEW.
Development of the human gastrointestinal microbiota and insights from high-throughput sequencing.
Succession of microbial consortia in the developing infant gut microbiome.

